Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Phytoremediation: A Sustainable and Green Approach for the Removal of Heavy Metals from Environment by Mangrove (Avicenna. marina)
*Corresponding author: Vibha Bhardwaj, Director Environment Laboratories, Ras Al Khaimah Municipality, Ras Al Khaimah, United Arab Emirates.
Received: July 30, 2024; Published: August 05, 2024
DOI: 10.34297/AJBSR.2024.23.003093
Annotation
Introduction: Toxic metal pollution is a universal problem. One of the most rapidly growing ecologically beneficial and lucrative technologies is to use metal-accumulating plants to clean contaminated ecosystems. Nature-based remediation of pollutants is one of the most promising eco-friendly approaches for sustainable ecosystem management.
Objective: In the present research work, samples of soil sediment, root, stem, leaves of mangrove species Avicenna marina was collected from Ras Al Khaimah and Umm Al Quwain with the aim of evaluating heavy metal concentrations, Extraction Coefficient (EF) bioaccumulation factors (BAFshoots and BAFroots) and Translocation Factor (TF) to ensure soil contamination and phytoremediation potentiality.
Results and Discussion: Overall, the heavy metals concentration in soil sediment of Ras al Khaimah are Al:3104-3282mg/kg; Zn:104mg/kg; Fe:2782-2929mg/kg; Mn:68.80-70.36mg/kg; Sr:4472-5174mg/kg, Ca:182500-210000mg/kg, K:2238-2540mg/kg, Na:26740-29160mg/kg. The mean concentration of each metal in the plant tissue (root) was found following Al:17.99mg/kg; Zn: 16.53mg/kg; Fe:460.2mg/kg; Mn:3.46mg/kg; Sr:49.62mg/kg, Ca:1857mg/kg, K:2801mg/kg, Na:3437mg/kg. whereas, in the leaf part, the mean concentration (mg/kg) of each metal found as Al:15.93mg/kg; Zn:15.16mg/kg; Fe:23.51mg/kg; Mn:15.27mg/kg; Sr:51.44mg/kg, Ca:1660mg/kg, K:4452mg/kg, Na:6467mg/kg. Phytoremediation potentiality of the species was assessed by Bioaccumulation Factor (BAF), Extraction Coefficient (EF) and Translocation Factor (TF). BAF values in Ras Al Khaimah showed less accumulation for most of the heavy metals (<1) except K which was highly accumulated in all mangrove plants. However, BAF values in Umm al Quwain showed less accumulation for most of the heavy metals (<1) except K and Na which was highly accumulated in all mangrove plants. The Translocation Factor (TF) values depicted that most of the heavy metals were strongly translocated in plant (>1). However, the BAF value depicts that K was highly bioconcentrated in Avicenna marina.
Conclusion: Heavy metals, Bioaccumulation factors, Translocation factors, Avicenna marina, Phytoremediation.
Abbreviations: PSD: Standard deviation; TF: Translocation factor; EC: Extraction coefficient; BAF: Bioaccumulation factor; RAK: Ras Al Khaimah; UAQ: Umm Al Quwain
Introduction
Currently the world is facing many challenges, and heavy metal pollution is one of them. Many heavy metals play essential role for plant growth as micronutrients are not toxic (e.g., Co, Cu, Fe, Mn, Mo, Ni, Al, Rb, Ti, and Zn) until they go beyond a certain limit [1,2]. There are non-essential heavy metals which are toxic to plant growth (e.g., Cd, Pb, U, Cr, Ag, Hg, and Zr), while Arsenic and Selenium are metalloids are also toxic [3,4]. Heavy metals are toxic, bio-accumulative, not naturally biodegradable, persistent and can enter the food chain [5,6]. To degrade organic contaminants from environment, chemical treatments are relatively successful, but they are quite expensive and non-ecofriendly. This technique cannot be use for removing toxic heavy metals from the soil [6]. Therefore, there is a demand for the development or use of effective, affordable, and ecofriendly technology to rectify the problem. Sustainable idea of using green plants to eradicate or reduce the metal contaminants, known as phytoremediation, has been successful as a promising environmentally sustainable technology [7]. Plants are remarkably beneficial for bioremediation to prevent leaching and erosion that can spread the toxic substances to surrounding areas (USEPA, 2004). Plants generally control the pollutants without affecting topsoil, thus conserving its utility and fertility with inputs of organic matter [8].
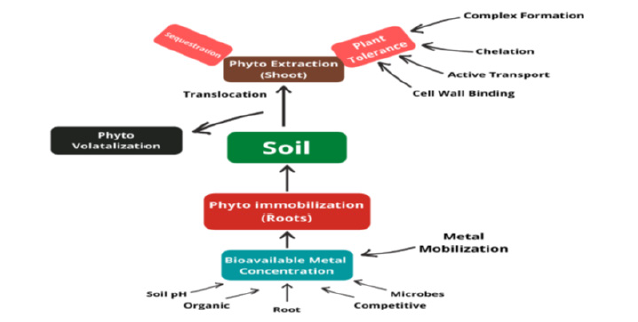
Mangrove sediments are thought to sequester toxic metals as several studies have demonstrated that reforestation has improved reduction of metals from water and surrounding environments [9,10]. For industrialization and urbanization, huge amount of metal waste is discharged into the coastal ecosystems [9,10]. Heavy metals expelled into coastal ecosystems because of human activities are frequently associated with particulate matter, which settles and becomes deposited in sediments [11]. A high concentration of Heavy metals in sediment are absorbed by organisms and retained in their tissues, affecting biological responses and hindering growth and development mechanisms [12]. As a result, coastline sediments are interpreted to be important markers for determining the health of ecosystems [13]. Therefore, understanding the distribution of heavy metals including the toxic one, and monitoring their potential bioavailability to mangrove plants have become increasingly important [14]. Recent studies have recognized the incidence and severity of sediment contamination by heavy metals in mangrove ecosystems [15,16]. Pollutants are released through industrial activities and eventually enter aquatic ecosystems [16]. The foremost contaminant metals are Cu, Mn, Ni, Zn, Pb, Cd, and As, which have been revealed in significant concentrations in coastal and inshore waters [17,18] (Figure 1).
Mangroves are one of the most beneficial biomes on the planet, providing a wide range of services such as animal feeding and habitat, erosion mitigation and coastal landform stabilization [19]. These plants or salt marshes grow in coastal sediments, and providing a medium of biological absorption and can modify the rate of heavy metal adsorption for phytoremediation purposes [20,21]. Mangroves are also characterized as “green barriers” because of their exceptional capacity to reduce metal transmission to nearby environments [18]. In addition, metal concentrations in leaves reflect those in the soil and the environment, justifying its use as bio-indicators [22,23]. However, the translocation of metals from the soil to mangrove leaves, may result in the remobilization of heavy metals stored in the soil during detritus senescence. Although this will lead to a reduction of heavy metals in soils. Despite pollution in mangroves have been widely studied [24-27] and previous studies showed that mangroves have the capacity to reabsorb nutrients before shedding the leaves [28-30] (Figure 2).
The present investigation is to assess the phytoremediation potential of mangrove plants growing on metal enriched sediments. Different parts of the mangrove plant were assessed for heavy metal uptake, accumulation and remediation. The aims of this study are: (i) To determine the concentration of heavy metals in mangrove sediment and plants; (ii) to estimate the accumulation and translocation ability of heavy metals in A. marina mangrove plants. The hypothesis is mangrove plants are highly potential to remove or accumulate heavy metals. This study will help to recognize the A. marina species for phytoremediation.
Material and Methods
Site Description
Samples were collected from two different Emirates of United Arab Emirates. The research sample collection area from Emirate of Ras Al Khaimah was Tower links Golf course, Qurm and Khuzam Road and sample’s location of Emirate of Umm Al Quwain was from beach (border of Umm Al Quwain and Ras Al Khaimah).
Sample Collection, Preparation and Analysis
Sediment and plants samples were collected from February to April 2024. A total of six surface sediment samples (two samples from each location) were taken from top 0-10cm (recently deposited sediment), which covers an area of 1m2. Moreover, Avicenna marina species of mangrove trees was selected for collecting samples, because of their dominancy in the study area and have not studied yet in Ras Al Khaimah, United Arab Emirates for their phytoremediation potentiality. Without posing any detrimental effect to the plant, 6 plant samples (shoot and roots) were obtained [21]. Leaves and roots were cut off with a sharp sterilised knife, thoroughly rinsed to remove any clinging dirt and placed in a zip lock plastic bag before being transported to the laboratory.
After collection, the soil sediment samples were sieved in the laboratory to remove debris. After that, each sample placed in oven at temperature (~45°C) [31] until completely dried, as high temperature may influence to the alteration of volatile and even non-volatile organics of the sample [32], until soil sample get fully dried. Samples were sieved through 63μm mesh. Then, individually transferred to vessel tube with conc. 2% HNO3 for microwave digestion. Leaf, stem and root tissue was oven-dried at 80°C for 2-3 days [33]. Tissue was subsequently ground to a fine powder and sieved through a 2-mm mesh nylon sieve. One gram of plant tissue was placed in each vessel tube with conc. 2% HNO3 for microwave digestion (CEM MARS Shimadzu.). The digested samples of soil, shoot and root were tested for concentration of heavy metals by ICPOES (Analytik Jena PQ9000).
Assessment of Phytoremediation Potentiality
The ability of plants to withstand and accumulate heavy metals could be used for phytoextraction and bioremediation of the metal-contaminated area. In contrast, BAF and TF can be used to estimate a plant’s phytoremediation capacity [34]. Pollutants accumulate in the plant because the increased contaminants it absorbs are not processed fast [35]. The potential of native plants to undertake phytoremediation can be determined by comparing their Bioaccumulation Factor (BAF) and Translocation Factor (TF). BAF was calculated using the following two equations to determine the phytoextraction capabilities of the plants investigated [36].
Three internationally recognized hyperaccumulator indicators were used to evaluate the hyperaccumulator species listed as follows:
Bioaccumulation Factor (BAF): The bioaccumulation factor is the capability of a plant to accumulate metal from soils. It is defined as the ratio of metal concentration in the shoot to the metal concentration in the rhizosphere soil [37]. This value reflects the progressive accumulation of metal in the plant [38]. The bioconcentration factor for metals was calculated as follows:
BAF leaf=C leaf/C sediment
BAF bark=C bark/C sediment
BAF root=C root/C sediment
where Cleaf, Cbark and Croot are the trace metal concentrations in the leaf, bark and root, respectively, and Csediment is the extractable concentration of trace metal in the sediment. It is used for quantitative analysis of accumulation.
Translocation Factor (TF): The TF is the capacity of a plant to transfer metal from its roots to shoots. The Translocation Factor (TF) for each plant was calculated by dividing metal concentration in the shoot by metal concentration in the root. A TF value>1 indicates the plant’s capability to translocate metal effectively from root to shoot [37]. The equation was as follows:
TF leaf=Cleaf/Croot
where Cleaf and Croot are the trace metal concentrations in the leaf and root, respectively [39,40]. A translocation factor greater than 1 indicates preferential partitioning of metals to the shoots [32].
Extraction Coefficient (EF): It evaluates the ability of the plant to accumulate heavy metals in shoot biomass [36] and extraction coefficient more than 1 is one of the criteria for identifying hyperaccumulator plants [41]. The equation was as follows:
EF=Cshoot/Csediment
Statistical Analysis
Experiments performed in triplicate. Data are expressed as mean. Experimental error was determined for triplicate and expressed as Standard Deviation (SD).
Results and Discussion
According to the research results, this is possibly the first report to study about phytoremediation potential of A. marina in Ras Al Khaimah, United Arab Emirates. In the present research work, ICPOES was used for heavy metal analysis.
Metal Concentrations in Sediment
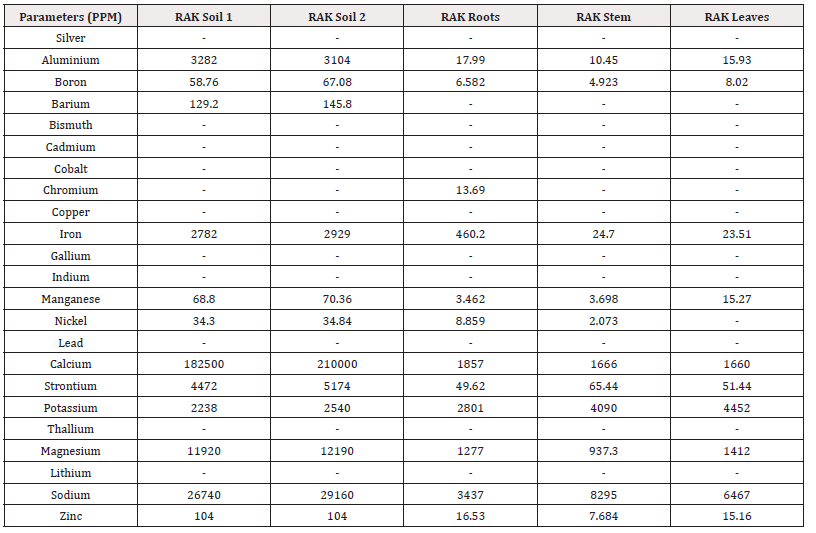
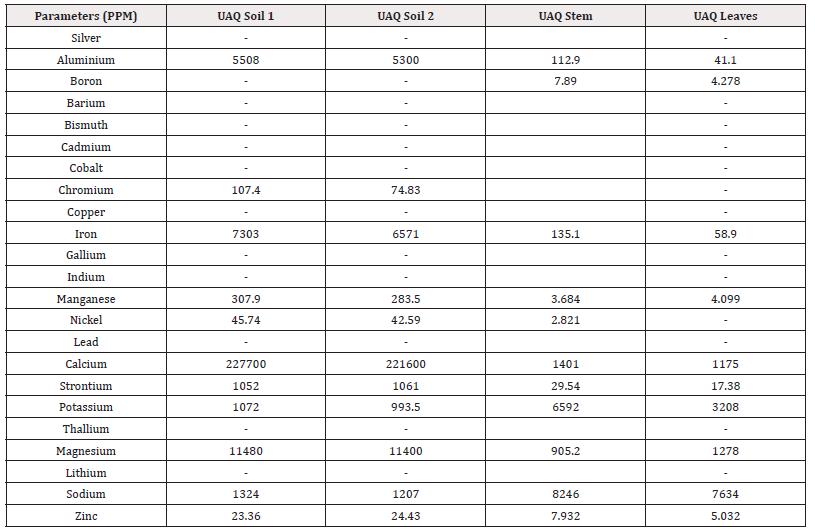
The present study analysed 23 metals from the mangrove sediments. Concentrations of heavy metals in mangrove soil of Ras Al Khaimah ranged as follows; Al:3104-3282mg/kg; Zn:104mg/kg; Fe:2782-2929mg/kg; Mn:68.80-70.36mg/kg; Sr:4472-5174mg/kg, Ca:182500-210000mg/kg, K:2238-2540mg/kg, Na:26740-29160mg/kg, Mg:11920-12190mg/kg (Table 1). Moreover, the concentration of heavy metal in mangrove soil of Umm Al Quwain as follows Al:5300-5508mg/kg; Zn:23.36-24.43mg/kg; Fe:6571-7303mg/kg; Mn:283.5-307.9mg/kg; Sr:1052-1061mg/kg, Ca:221600-227700mg/kg, K:993.5-1072mg/kg, Na:1207-1324mg/kg.
However, Na, Mg and Ca concentrations were found higher in Ras Al Khaimah (Table 1) than the UAQ. Among the studied metals Fe, Ca, Mg and Al concentrations were found higher in UAQ (Table 2).
Magnesium and calcium, occurs naturally in water bodies, are among the most highly available alkali metals in the environment [42]. Magnesium salts are found naturally and in high concentrations in surface and ground water, and the only other elements that occur in greater abundance are sodium and calcium cations. Magnesium and calcium concentrations in ground and surface waters increase as those elements are washed out from bedrock [43].
Concentration of Metals in Mangroves
Trace metals are absorbed by plants through their roots, branches and leaves and store them in various plant parts. The distribution and accumulation of trace metals are influenced by plant types, metal sources and sediment metal concentrations [44]. In the analysed mangrove species, the mean concentration in Ras Al Khaimah of each metal in the plant tissue (root) was found following Al:17.99mg/kg; Zn:16.53mg/kg; Fe:460.2mg/kg; Mn:3.46mg/kg; Sr:49.62mg/kg, Ca:1857mg/kg, K:2801mg/kg, Na:3437mg/kg. In leaves, the mean concentration (mg/kg) of each metal found as Al:15.93mg/kg; Zn:15.16mg/kg; Fe:23.51mg/kg; Mn:15.27mg/kg; Sr:51.44mg/kg, Ca:1660mg/kg, K:4452mg/kg, Na:6467mg/kg (Table 1). However, the metal concentration in UAQ mangrove plant leaves were found as Al:41.10mg/kg; Zn:5.032mg/kg; Fe:58.90mg/kg; Mn:4.099mg/kg; Sr:17.38mg/kg, Ca:1175mg/kg, K:3208mg/kg, Na:7634mg/kg, Mg:1278mg/kg (Table 2). Particularly, Na and K concentrations were higher in the roots and leaves of A. marina of RAK and UAQ. The concentrations of Zn were almost similar in roots and leaves, but for the Sr the concentrations were higher in leaves than the roots of all plants in RAK (Table 1). Chowdhury R, et al., [45] reported that the concentration of Fe in mangrove tissue of all the plants was the highest and Mn showed the second highest concentrations than the other heavy metals of mangroves of India. The variation of specific metal accumulation depends on their individual physiological rhythms and prevailing ecological conditions of the inhabiting environment [46,47]. For example, in acid-sulphate soil, the Fe concentrations are higher and resulted in higher accumulation of this metal in mangrove plants [45].
Phytoremediation Potentiality of Mangroves
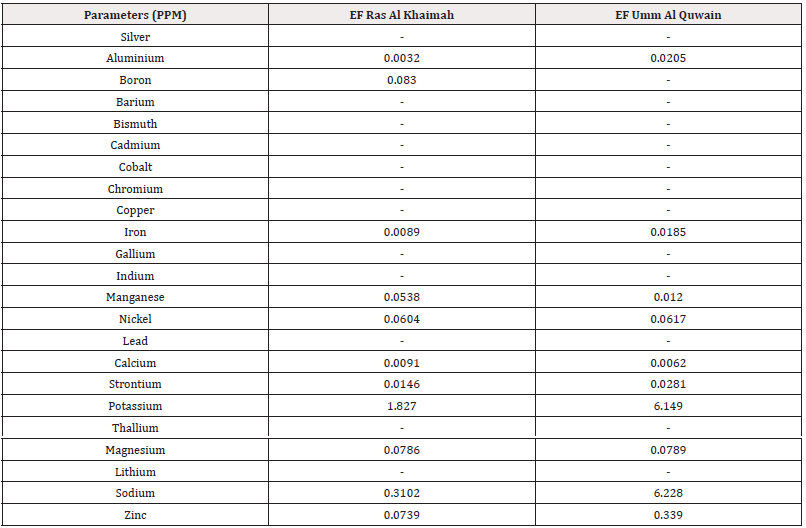
Bioaccumulation Factor (BAF): The Bioaccumulation factor (BAF) from sediment to various body parts (root and leaf) of the mangrove species Avicennia marina was used as an indicator of species accumulation ability from nature and is calculated as the proportion of metal concentration in plant tissue and sediment. This study assumed that plants with BAFshoot values>1 are accumulators, while plants with BAFshoot values<1 are excluders [48]. Additionally, plants were classified as potential hyperaccumulators if the BAFshoot values were>10 [49]. The values of BAF for 23 metals from sediment to roots and leaves of the mangrove species were calculated in this study (Table 3). The results showed that all the plant parts (roots and leaves) had BAF values<1 except potassium and sodium. However, the BAF value>1 for K was found in both roots, stem and leaves of mangrove species of Ras Al Khaimah. Overall, the highest BAF value (6.228) was found for Na in the stem of Avicennia marina of UAQ. [50,45] also reported same BCF value for mangrove plants in their studies in India.
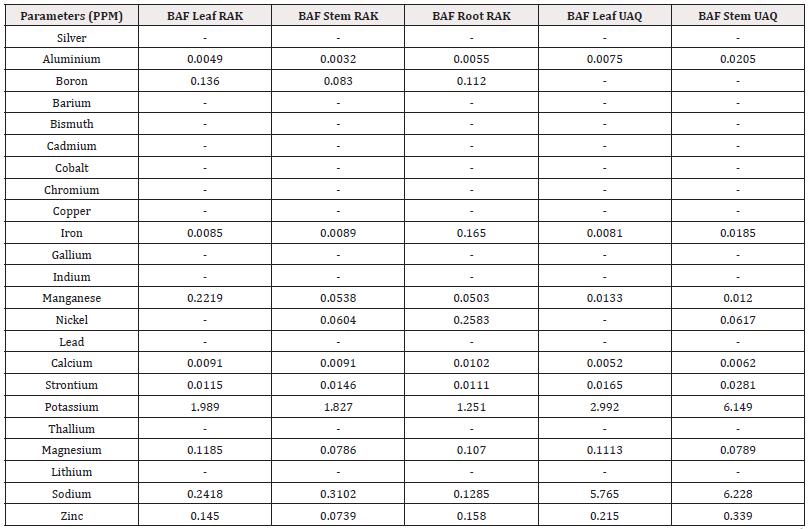
Table 3: Bioaccumulation Factor (BAF) Ras Al Khaimah (RAK) and Umm Al Quwain (UAQ).
*Note: BAF leaf =C leaf/C sediment; BAF bark=C bark/C sediment; BAF root=C root/C sediment where Cleaf, Cbark and Croot are the trace metal concentrations in the leaf, bark and root,respectively, and Csediment is the extractable concentration of trace metal concentration in the sediment.
Our results showed that A. marina had BAFshoot values>1, indicating that they had the potential for use as accumulators or hyperaccumulators of K. The success of the phytoextraction process depends on heavy metal removal by the shoots [39]. Therefore, we suggested that the plant species having the higher metal concentrations in their shoots than in their roots can be considered as accumulators or hyperaccumulators for phytoremediation.
Translocation Factor (TF): The Translocation Factor (TF) is required for a detailed explanation of entire scenario of metal accumulation in the plant body [48,51,34,39]. TF is defined as the ratio of the metal concentration in the shoots to that in the roots. Plants with TF values>1 is classified as high-efficiency plants for metal translocation from the roots to shoots [49]. Tables 4 show the TF of A. marina of Ras Al Khaimah which shows highest values are Mn4.4107, Na1.881, K1.589, B1.218, Sr1.0367, Zn0.917, Ca0.8939, based on metal concentration ratios in the leaf and root of mangrove species of Ras Al Khaimah (Table 4). TF values were found>1 for maximum metals except Fe, Ca and Zn in the studied plants which mean these plants can actively take up trace metals from the sediment and are able to accumulate them in their aerial parts, as a result can be good phytostabilisers. This result was very similar with the investigation of [45], for Indian mangroves.
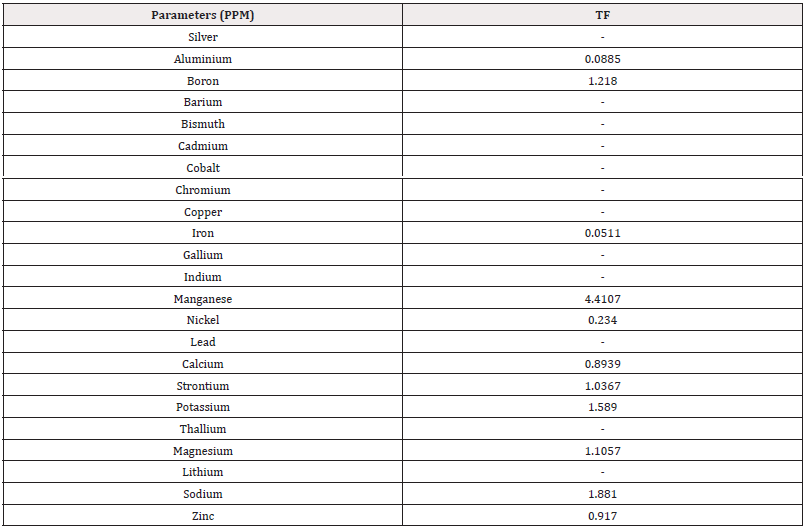
Table 4: Translocation Factor (TF) Ras Al Khaimah.
*Note: TFleaf=Cleaf/Croot, where Cleaf and Croot are the trace metal concentrations in the leaf and root, respectively. A translocation factor greater than 1 indicates preferential partitioning of metals to the shoots (Usman et al., 2009; Usman et al., 2012).
Similar results were found by [52] who observed significant metals mobilization between the plant parts above and below the surface of the sediment with translocation factor (TF)>1. This indicates that the plant translocates elements effectively from root to the shoot. According to [45] translocation factor values shows that S. apetala exhibited high values for Mn (4.48 and 31.99), Zn (9.95, 3.25) and Cu (3.42, 3.47) and Pb (1.84, 18.01) for Jharkhali (S1) and Gangadharpur (S2), respectively.
Extraction Coefficient Factor (EF): It evaluates the ability of the plant to accumulate heavy metals in shoot biomass [36] and extraction coefficient more than 1 is one of the criteria for identifying hyperaccumulator plants [41]. According to our research findings, in case of Ras Al Khaimah mangrove sample, K1.827 showed highest extraction coefficient and Na0.3102 second highest. However, in case of UAQ mangrove plant sample Na6.228 showed the highest extraction coefficient then K6.149 and then Zn0.339 respectively (Table 5).
Conclusion
This study concluded that the A. marina mangrove plant species has phytoremediation capacity. Our results revealed that, plant species of A. marina as a hyperaccumulator, and is appropriate for phytoextraction of heavy metals from contaminated soils. However, in the case of TF, all the plants exhibited values greater than one for most of the metals, indicating that these plants can translocate metals from root to leaf and may operate as a phytoremediator in the study region. Taken together, our these findings indicate that phytoremediation may provide a sustainable option to remediate heavy metal contaminated soils, by A. marina mangrove plant. Future studies are necessary to evaluate the phytoremediation efficacy of identified plant species against various types and concentrations of metals, and to investigate the mechanisms of phytoextraction of heavy metals [53-55].
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Materials
The relevant data and materials are available in the present study.
Competing Interests
The authors declare that they have no competing interests. All procedures followed were in accordance with the ethical standards (institutional and national).
Funding
Not applicable.
Acknowledgements
Authors would like to thank all individuals who provided their efforts for this research especially Aaesha Ahmed Alzaabi, Nizamudeen and Imran for their assistance during research work.
Author’s Contributions
VB performed all the experiments. VB analysed the data and wrote the manuscript.
References
- Appenroth KJ (2010) What are “heavy metals” in plant sciences? Acta Physiologiae Plantarum 32: 615-619.
- Kabata Pendias A (2010) Trace Elements in Soils and Plants. Boca Raton: 548.
- Bothe H (2011) “Plants in heavy metal soils,” in Detoxification of Heavy Metals, eds S. Irena and V. Ajit (Berlin; Chicago, IL; Heidelberg: Springer): 35-57.
- Shahid M, Ferrand E, Schreck, E, Dumat C (2013) Behavior and impact of zirconium in the soil-plant system: plant uptake and phytotoxicity. Rev Environ Contam Toxicol 221: 107-127.
- Li P, Li X, Bai J, Meng Y, Diao X, et al. (2022) Effects of land use on the heavy metal pollution in mangrove sediments: Study on a whole island scale in Hainan, China. Sci Total Environ 824: 153856.
- Zhu G, Noman MA, Narale DD, Feng W, Pujari L, et al. (2020) Evaluation of ecosystem health and potential human health hazards in the Hangzhou Bay and Qiantang Estuary region through multiple assessment approaches. Environ Pollut 264: 114791.
- Khan S, Afzal M, Iqbal S, Khan QM (2013) Plant-bacterial partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere 90(4): 1317-1332.
- Mench M, Schwitzguebel JP, Schroeder P, Bert V, Gawronski S, et al. (2009) Assessment of successful experiments and limitations of phytotechnologies: contaminant uptake, detoxification and sequestration, and consequences for food safety. Environ Sci Pollut Res Int 16: 876-900.
- Hwang DW, Kim SG, Choi M, Lee IS, Kim SS, et al. (2016) Monitoring of trace metals in coastal sediments around Korean Peninsula. Mar Pollut Bull 102(1): 230-239.
- Rahman MS, Ahmed Z, Seefat SM, Alam R, Islam ARMT, et al. (2022) Assessment of heavy metal contamination in sediment at the newly established tannery industrial Estate in Bangladesh: A case study. Environmental Chemistry and Ecotoxicology 4: 1-12.
- Zhang C, Shan B, Tang W, Dong L, Zhang W, et al. (2017) Heavy metal concentrations and speciation in riverine sediments and the risks posed in three urban belts in the Haihe Basin. Ecotoxicol Environ Saf 139: 263-271.
- Signa G, Mazzola A, Di Leonardo R, Vizzini S (2017) Element-specific behaviour and sediment properties modulate transfer and bioaccumulation of trace elements in a highly-contaminated area (Augusta Bay, Central Mediterranean Sea). Chemosphere 187: 230-239.
- Islam MS, Hossain MB, Matin A, Sarker MSI (2018) Assessment of heavy metal pollution, distribution and source apportionment in the sediment from Feni River estuary, Bangladesh. Chemosphere 202: 25-32.
- Silva CAR, Rainbow PS, Smith BD (2003) Biomonitoring of trace metal contamination in mangrove-lined Brazilian coastal systems using the oyster Crassostrea rhizophorae: comparative study of regions affected by oil, salt pond and shrimp farming activities. Hydrobiologia 501(1): 199-206.
- Anouti FA (2014) Bioaccumulation of heavy metals within mangrove ecosystems. Biodiversity and Endangered species 2(2): 1-2.
- Ratheesh Kumar CS, Joseph MM, Gireesh Kumar TR, Renjith KR, Manju MN, et al. (2010) Spatial variability and contamination of heavy metals in the inter-tidal systems of a tropical environment. International Journal of Environmental Research 4(4): 691-700.
- Kapi C, Patient G, Nelly K, Patrick EA, Rodrigue A (2011) Evaluation of heavy metals pollution of Nokoue Lake. African Journal of environmental science and technology 5(3): 255-261.
- Machado W, Moscatelli M, Rezende L, Lacerda L (2002) Mercury, zinc, and copper accumulation in mangrove sediments surrounding a large landfill in southeast Brazil. Environ Pollut 120(2): 455-461.
- Choudhury TR, Acter T, Uddin N, Kamal M, Chowdhury AS, et al. (2021) Heavy metals contamination of river water and sediments in the mangrove forest ecosystems in Bangladesh: A consequence of oil spill incident. Environmental Nanotechnology Monitoring and Management 16(2): 100484.
- Rahman MS, Hossain MB, Babu SOF, Rahman M, Ahmed AS, et al. (2019) Source of metal contamination in sediment, their ecological risk, and phytoremediation ability of the studied mangrove plants in ship breaking area, Bangladesh. Mar Pollut Bull 141: 137-146.
- Chowdhury R, Favas PJ, Pratas J, Jonathan M, Ganesh PS, et al. (2015) Accumulation of trace metals by mangrove plants in Indian Sundarban Wetland: Prospects for phytoremediation. Int J Phytoremediation 17(9): 885-894.
- Murray F (1985) Cycling of fluoride in a mangrove community near a fluoride emission source. J Appl Ecol 22: 277-285.
- Pinheiro MAA, e Silva PPG, de Almeida Duarte LF, Almeida AA, Zanotto FP (2012) Accumulation of six metals in the mangrove crab Ucides cordatus (Crustacea: Ucididae) and its food source, the red mangrove Rhizophora mangle (Angiosperma: Rhizophoraceae). Ecotoxicol Environ Safety 81: 114-121.
- Wang Y, Qiu Q, Xin G, Yang Z, Zheng J, et al. (2013) Heavy metal contamination in a vulnerable mangrove swamp in South China. Environ Monitor Assess 185(7): 5775-5787.
- Zhang ZW, Xu XR, Sun YX, Yu S, Chen YS, et al. (2014) Heavy metal and organic contaminants in mangrove ecosystems of China: a review. Environ Sci Pollut Res Int 21(20): 11938-11950.
- Alzahrani DA, Selim EMM, El Sherbiny MM (2018) Ecological assessment of heavy metals in the grey mangrove (Avicennia marina) and associated sediments along the red sea coast of saudi arabia. Oceanologia 60(4): 513-526.
- Kulkarni R, Deobagkar D, Zinjarde S (2018) Metals in mangrove ecosystems and associated biota: a global perspective. Ecotoxicol Environ Saf 153: 215-228.
- Alongi D, Clough B, Robertson A (2005) Nutrient-use efficiency in aridzone forests of the mangroves Rhizophora stylosa and Avicennia marina. Aquatic Botany 82(2): 121-131.
- Zhou HC, Wei SD, Zeng Q, Zhang LH, Tam NF, et al. (2010) Nutrient and caloric dynamics in Avicennia marina leaves at different developmental and decay stages in Zhangjiang River Estuary, China Estuarine Coastal and Shelf Science 87(1): 21-26.
- Almahasheer H, Duarte CM, Irigoien X (2018) Leaf nutrient resorption and export fluxes of Avicennia marina in the Central Red Sea area. Front Mar Sci 5: 204.
- Watts MJ, Barlow TS, Button M, Sarkar SK, Bhattacharya BD, et al. (2013) Arsenic speciation in polychaetes (Annelida) and sediments from the intertidal mudflat of Sundarban mangrove wetland, India. Environ Geochem Health 35(1): 13-25.
- Mudroch A, Azcue JM (1995) Manual of aquatic sediment sampling. CRC Press, Boca Raton.
- Bhardwaj V (2021) Avicennia Marina: A Novel Convivial Phyto Medicine for Antibiotic Resistant Pathogenic Bacteria. J Biomedical Studies 1: 101.
- Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368(2-3): 456-464.
- Chandra R, Yadav S, Yadav S (2017) Phytoextraction potential of heavy metals by native wetland plants growing on chlorolignin containing sludge of pulp and paper industry. Ecological Engineering 98: 134-145.
- Phaenark C, Pokethitiyook P, Kruatrachue M, Ngernsansaruay C (2009) Cd and Zn accumulation in plants from the padaeng zinc mine area. Int J Phytoremediation 11(5): 479-495.
- Rezvani M, Zaefarian F (2011) Bioaccumulation and translocation factors of cadmium and lead in Aeluropus littoralis. Australian Journal Agricultural Engineering 2(4): 114-119.
- Branquinho C, Serrano HC, Pinto MJ, Martins Loucao MA (2007) Revisiting the Plant Hyperaccumulation Criteria to Rare Plants and Earth Abundant Elements. Environ Pollut 146(2): 437-443.
- Usman ARA, Mohamed HM (2009) Effect of microbial inoculation and EDTA on the uptake and translocation of heavy metals by corn and sunflower. Chemosphere 76(7): 893-899.
- Usman ARA, Lee SS, Awad YM, Lim KJ, Yang JE, et al. (2012) Soil pollution assessment and identification of hyperaccumulating plants in chromated copper arsenate (CCA) contaminated sites, Korea. Chemosphere 87: 872-878.
- Chen Y, Shen Z, Li X (2004) The use of vetiver grass (Vetiveria zizanioides) in the phytoremediation of soils contaminated with heavy metals. Applied Geochemistry 19(10): 1553-1565.
- Grochowska J, Tandyrak R (2009) The influence of the use of land on the content of calcium, magnesium, iron and manganese in water exemplified in three lakes in Olsztyn vincinity. Limnological Review 9(1): 9-16.
- Gałczyńska M, Gamrat R, Burczyk P, Horak A, Kot M (2013) The influence of human impact and water surface stability on the concentration of selected mineral macroelements in mid-field ponds. Water Environment Rural Areas 3(3/43): 41-54.
- Seleiman MF, Selim S, Jaakkola S, Mäkelä PS (2017) Chemical composition and in vitro digestibility of whole-crop maize fertilized with synthetic fertilizer or digestate and harvested at two maturity stages in boreal growing conditions. Agriculture Food Science 26(1): 47-55.
- Chowdhury R, Lyubun Y Favas PJ Sarkar SK (2016) Phytoremediation potential of selected mangrove plants for trace metal contamination in Indian Sundarban wetland. Springer International Publishing pp: 283-310.
- Thomas G, Fernandez TV (1997) Incidence of heavy metals in the mangrove flora and sediments in Kerala, India. In Proceedings of the Asia-Pacific Conference on Science and Management of Coastal Environment, Hong Kong, 25-28 June 1996; Springer: Dordrecht, The Netherlands pp: 77-87.
- Kumar M, Mohapatra S, Karim AA, Dhal NK (2021) Heavy metal fractions in rhizosphere sediment vis-à-vis accumulation in Phoenix paludosa (Roxb.) mangrove plants at Dhamra Estuary of India: Assessing phytoremediation potential. Chemistry and Ecology 37(1): 1-14.
- Baker AJM (1981) Accumulators and excluder-strategies in the response of plants to heavy metals. J Plant Nutr 3(1-4): 643-654.
- Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, et al. (2001) A fern that hyperaccumulates arsenic. Nature 409(6820): 579.
- Agoramoorthy G, Chen FA, Hsu MJ (2008) Threat of heavy metal pollution in halophytic and mangrove plants of Tamil Nadu, India. Environ Pollut 155(2): 320-326.
- Srivastava M, Ma LQ, Santos JAG, (2006) Three new arsenic hyperaccumulating ferns. Sci Total Environ 364(1-3): 24-31.
- Sinegani AAS, Ebrahimi P (2007) The potential of Razan-Hamadan highway indigenous plant species for the phytoremediation of lead contaminated land. Soil Environ 26: 10-14.
- Lee SB, Lee YB, Lee CH, Hong CO, Kim PJ, et al. (2008) Characteristics of boron accumulation by fly ash application in paddy soil. Bioresour Technol 99(13): 5928-5932.
- (2004) Hazard summary. Lead compounds. United States Environmental Protection Agency (USEPA).
- Verma RK, Sankhla MS, Ekta B Jadhav, Parihar K, Awasthi KK (2022) Phytoremediation of Heavy Metals Extracted from Soil and Aquatic Environments: Current Advances as well as Emerging Trends. Biointerface Research in Applied Chemistry 12(4): 5486-5509.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.