Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
LDL Quantity and Quality in Cardiovascular Disease Risk Prediction
*Corresponding author: Rodney G Bowden, Professor and Brown Foundation Endowed Chair, Department of Public Health, Baylor University, USA.
Received: August 06, 2024; Published: August 13, 2024
DOI: 10.34297/AJBSR.2024.23.003105
Abstract
Cardiovascular disease remains the leading cause of mortality globally, with a notable increase in prevalence from 271 million in 1990 to 523 million in 2019. In the U.S., CVD resulted in 931,578 deaths in 2021, with related expenses reaching $422.3 billion in 2020. Central to CVD pathology is Low-Density Lipoprotein (LDL), which is implicated in the development of atherosclerosis, a major cause of stroke and ischemic heart disease. Despite widespread use of LDL-focused lipid-lowering therapies, recent studies have revealed inconsistencies in the association between LDL levels and cardiovascular mortality. This review examines the limitations of traditional LDL measurement and explores alternative predictors for cardiovascular risk. The historical context of LDL research underscores its role in atherogenesis, while advancements in lipoprotein analysis have introduced new metrics such as LDL particle number, apolipoprotein B100, and LDL particle size and number. These newer parameters have potential in offering more precise risk assessments compared to traditional LDL cholesterol measurements. LDL particle number and Apo-B100 have emerged as indicators of cardiovascular events and mortality. Additionally, small dense LDL and the LDL-C/ApoB ratio have been identified as promising tools for predicting cardiovascular risk. This review highlights that quantifying LDL cholesterol to evaluating LDL quality and its various subtypes may be needed for clinicians to help diagnose and treat cardiovascular disease.
Keywords: Low density lipoprotein, LDL, LDL quality, LDL quantity, LDL particle number, LDL size
Introduction
Cardiovascular Disease (CVD) continues to be the leading cause of death in most countries, with the global prevalence of cardiac patients rising substantially from 271 million in 1990 to 523 million in 2019, representing an almost twofold increase [1]. For over a decade, CVD has been the leading cause of death in the United States (US), causing 931,578 deaths in 2021 alone and the combined expenses for managing and treating CVD in the US in 2020 amounted to $422.3 billion or about $1,300 per person [2]. CVD, particularly Ischemic Heart Disease (IHD) and stroke are the leading causes of mortality and morbidity worldwide, with the formation of atherosclerotic plaque being the primary cause of stroke and ischemic heart disease [3]. Low-Density Lipoprotein (LDL) is widely recognized as the causal factor in the development of atherosclerosis [4]. Alloubani, et al. in their systematic review, observed a consistent positive association between LDL cholesterol levels and the occurrence of coronary heart disease, as evidenced by most observational studies conducted in various populations [5]. Research has demonstrated that an elevated level of LDL cholesterol significantly increases the risk of stroke and myocardial infarction even in the 70-100-year age group [6]. In this context, all heart disease guidelines advocate the use of lipid-lowering medications with the primary objective of reducing LDL levels, and this reduction is considered a critical goal in the treatment and prevention of both stroke and cardiovascular disease [7,8]. "The American College of Cardiology and the American Heart Association recommend checking the lipid profile in stable patients receiving lipid-lowering drugs every four to twelve months, depending on the patient's status [9]. Additionally, the guidelines recommend increasing the frequency of lipid profile testing to every 6 to 12 weeks when initiating or modifying lipid-lowering drug therapy [9]. A standard lipid profile includes total cholesterol, LDL cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides [10]. LDL is the primary focus of lipid management due to its detrimental effect on the mortality and morbidity of cardiovascular disease and stroke [11]. The widespread use of lipid-lowering drugs, particularly statins, has significantly reduced LDL cholesterol levels, sometimes even to below 30 mg/dL [12]. Studies have demonstrated that achieving a very low level of LDL (<30 mg/dL) is beneficial in reducing cardiovascular risk [13-15].
Although physicians aim to lower LDL, low LDL is found to be independently associated with increasing all-cause mortality and non-CVD mortality rates in critically ill patients [16]. Despite the prevailing view that LDL is the primary etiological agent for CVD, A Systematic Review and Meta-analysis show that comprehensive approaches targeting the reduction of LDL levels have not consistently established a proportional risk reduction in mortality due to CVD [17]. A meta-analysis including twenty-one studies found no conclusive association between lowering LDL-C levels and lowering CVD-related mortality risk [18].
The conflicting and inconsistent results from several studies have prompted scientists and researchers to consider factors beyond the quantity of LDL. In addition to a focus on quantity of LDL, scientists and clinicians are exploring better parameters to predict CVD risk and mortality. The composition of LDL, including its size, shape, protein content, and particle number, is gaining attention from scientists. This context underscores the growing necessity for a comprehensive understanding of current research findings regarding LDL and explaining the inconsistency of these findings. The objective of this review is to provide an overview of the most recent research on LDL, focusing on the constraints and inconsistencies associated with using LDL as a predictor of CVD and exploring the potential of alternative predictors that could better serve in predicting cardiovascular disease risk and mortality.
Previous LDL Research
The study of lipids in humans began in 1769 when François Poulletier de la Salle identified solid cholesterol in gallstones. Cholesterol was identified as a specific compound in 1815 by French chemist Michel Eugène Chevreul. In 1910, the German chemist Adolf Windaus discovered that human atherosclerotic plaques contained cholesterol reporting that the cholesterol content in atherosclerotic plaques of diseased human aortas was 25 times higher than that of normal aortas. This established the first link between illness and LDL. The Lipid Hypothesis is a medical theory that postulates a link between blood cholesterol levels and the occurrence of CVD. In 1913, Russian pathologist Nikolay Anichkov conducted a study reporting that rabbits who were fed on a high-cholesterol diet developed lesions in their arteries similar to atherosclerosis. This finding suggested a role for cholesterol in atherogenesis, the process of forming atheroma or plaques in the inner lining of arteries.
In 1929, Michel Machebeuf, working at the Pasteur Institute in Paris, suggested that circulating lipids existed in complexes with proteins. This was the first indication of the existence of lipoproteins. In 1955, John Gorman, a physician from the University of California, made a significant contribution to the field of lipidology by being the first to separate blood lipoproteins from blood plasma into Low-Density Lipoprotein (LDL) and High-Density Lipoprotein (HDL) [19]. And has been remembered as the father of clinical lipidology [20]. In 1972, researchers set out to unravel a human genetic disease called Familial Hypercholesterolemia (FH). In patients with FH, the concentration of cholesterol in the blood is elevated many times above normal, and heart attacks can occur early in life. The pioneering research on FH, a genetic disorder characterized by high cholesterol levels and premature cardiac events, was initiated by Joseph L Goldstein and Michael S Brown [21]. Expanding upon this research, it is worth noting that in 1973, Goldstein and Brown achieved a significant breakthrough by identifying the LDL receptor. This finding later served as the foundation for the effect of statins in lowering LDL levels. Finally, Gofman, et al. (1954) first described the association between elevated LDL levels and heart disease [21,22].
In 1981, a significant discovery was made by Alfred Alberts and his team. They demonstrated that statins augment the activity of LDL receptors in vivo. They discovered a fungal HMG CoA reductase inhibitor known as mevinolin, later renamed Mevacor or Lovastatin, because of their research. Researchers demonstrated that this compound enhances the activity of LDL receptors while simultaneously reducing plasma LDL levels [23]. In 1987, the first statin group of drugs was approved for human use. The Scandinavian Simvastatin Survival Study (4S) was the first large-scale clinical trial that demonstrated the benefits of statins in reducing the risk of heart attack and death in patients with coronary heart disease. The study was published in The Lancet in November 1994 [24].
Origin of LDL
The end product of fat digestion is free fatty acids and monoacylglycerols. These molecules reorganize in the intestinal cells to form triglycerides. Triglycerides (TG), along with cholesterol ester and vitamins, undergo chylomicron conversion, with triglycerides remaining the primary component, making up three-fourths of the chylomicron [25]. Lipid metabolism results in the formation of several lipoproteins with varied densities, including Low-Density Lipoproteins (LDL). Transporting, binding, and converting chylomicrons are crucial for this process [26]. The lymphatic system transports chylomicrons from the intestinal cells into the bloodstream25. During their transit in the bloodstream, they release free fatty acids and transform into chylomicron remnants25. Liver cells internalize these remnants, facilitating the binding of triglycerides through the action of microsomal Triglyceride Transfer Protein (MTP) [26]. This process results in the formation of very Low-Density Lipoprotein (VLDL) [26].
The enzyme lipoprotein lipase then converts VLDL into Intermediate-Density Lipoprotein (IDL) [27]. The enzyme lipoprotein lipase further converts IDL into LDL, most of which binds to LDL receptors in the liver [28]. This step is critical because it determines the cholesterol content delivered to peripheral tissues, which is a key factor in the development of atherosclerosis if dysregulated [28].
Composition of LDL
LDL is a heterogeneous compound composed of various molecules, including proteins and phospholipids29. Recent research suggests that the composition and content of LDL play a significant role in determining the risk of Cardio Vascular Disease (CVD) and mortality [30].
The nomenclature "low-density lipoprotein" is derived from its relatively low density, ranging from 1.019 to 1.063 g/mL, when compared to other lipoproteins, and LDL molecules, typically around 22 nm in diameter, are of varying size, shape, and composition [31]. LDL consists of TGs, Cholesteryl Ester (CE), proteins, and phospholipids, with the LDL molecule containing approximately 170 TG and 1600 CE at its center [32]. The outer surface of LDL contains 700 phospholipids, and each LDL molecule has one Apolipoprotein-B 100 (APO-B 100), which is one of the largest proteins in humans [32]. Almost 600 molecules of unesterified cholesterol are present in LDL; the majority of them (i.e., two-thirds) are found on the periphery of LDL, whereas the remainder (i.e., one-third) is found in the center [33]. Phospholipids, which also make up LDL, primarily include Phosphatidyl Choline (PC) and Sphingo Myelin (SM), with LDL consisting of 450 PC molecules and 185 SM molecules [33].
LDL Functions
Cholesterol is vital for human physiology as it is used to build the structure of cell membranes, makes hormones like estrogen, testosterone, and adrenal hormones, enables human metabolism to work efficiently, produces Vitamin D, and produces bile acids, which help the body digest fat and absorb important nutrients [34]. LDL serves as the principal carrier for delivering cholesterol to enhance various cellular functions. LDL transports about two-thirds of the endogenous cholesterol from the liver to the periphery inclusive of organs and tissues that contain LDL receptors, allowing them to recognize APO-B100 of LDL molecules. These organs and tissues include the testes, adrenal glands, SMC, and adipose tissues [35].
Role of LDL in Atherosclerosis
Atherosclerosis is the process by which various lipids and other chemicals accumulate within the large and medium arterial walls, leading to plaque development that ultimately causes arterial narrowing and reduced blood flow to the intended organ [36]. The initiation of atherosclerosis is triggered by arterial injury, which can be caused by factors such as inflammation, dyslipidemia, hyperglycemia, smoking, and hypertension [36]. LDL is a major causal factor for developing atherosclerosis, starting with the deposition of LDL in the tunica intima, the inner layer of the artery, which can stimulate both innate and acquired immunity-mediated inflammatory responses [37]. Inflammatory cells release enzymes that modify LDL molecules, which then activate macrophages to initiate immune cell infiltration through chemical mediators, leading to the phagocytosis of more LDL by macrophages and the formation of foam cells, which are prone to necrosis and apoptosis [38]. The core of atherosclerotic plaque is composed of foam cells and Smooth Muscle Cells (SMC), which undergo apoptosis and necrosis processes. Over time, the plaque undergoes a process of increased rigidity and subsequently may rupture [39].
LDL Subclasses and Measurement
The heterogeneity of LDL molecules was initially observed during the 1940s and 1950s [29]. Since these initial observations, a variety of analytical techniques have been employed to distinguish between different types of LDL, including ultracentrifugation, Gradient Gel Electrophoresis (GGE), Nuclear Magnetic Resonance (NMR), Vertical Auto Profile (VAP), Fast Protein Liquid Chromatography (FPLC), clinical chemistry methods, ion mobility analysis, and others [40]. In the context of correlating LDL subclasses with diseases, it is crucial to acknowledge that the density parameters of each LDL subclass may demonstrate slight discrepancies across various studies conducted for LDL subclass identification and this variability should be taken into account in such analyses [41].
Ultracentrifugation can classify LDL into four distinct subclasses, denoted as LDL-I (density 1.025-1.034 g/mL), LDL-II (LDL IIa and LDLIIb) (density 1.034-1.044 g/mL), LDL-III (LDL IIIa and LDL IIIb) (density 1.044-1.060 g/mL), and LDL-IV (LDL Iva and LDL IVb) (density >1.060 g/mL) [42]. The differentiation of these subclasses is determined by their diameter and density with progression from I-IV based decreasing diameter and increasing density [43]. Large LDL, often known as LDL I, has the greatest diameter and the least density and consequently, LDLIV, also known as extremely small LDL, possesses the smallest size and highest density [43].
Gradient Gel Electrophoresis (GGE), a method frequently used to separate LDL subclasses, effectively divides LDL into four distinct subclasses: LDL I (26.0-28.5 nm), LDL II (25.5-26.4 nm), LDL III (24.2-25.5 nm), and LDL IV (22.0-24.1 nm) [42]. Nuclear Resonance imaging (NMR) provides classified LDL based on the combination of their size as well as lipid mass [43]. NMR also categorizes LDL into three groups and the benefit of using NMR is the variation of results from NMR analysis vary less than 1% across the lab and thus it is reproducible [44].
Measurement of LDL-C
Standard lipid tests are designed to measure the amount of Low-Density Lipoprotein Cholesterol LDL-C in the bloodstream [45]. These tests typically provide values for triglyceride levels, total cholesterol, and HDL-C. Historically, the Friedewald equation, which is LDL-C = Total Cholesterol - HDL-C - (Triglycerides/5), was the accepted method for calculating LDL-C levels [45]. However, this equation has certain limitations as it tends not to provide accurate results when triglyceride levels are high or when LDL-C levels are low [46].
To address these issues, researchers at Johns Hopkins University developed a more precise method for calculating LDL-C, known as the Martin/Hopkins equation [47]. This equation calculates LDL-C using the formula: LDL-C = Total Cholesterol - HDL-C - (Triglycerides/Adjustable Factor) [47]. The adjustable factor in this equation varies based on the patient’s non-HDL-C and triglyceride levels, allowing for a more accurate estimation of LDL-C, especially in cases where LDL-C is low, and triglycerides are high [47]. Today, the Martin/Hopkins equation is widely recognized and used for calculating LDL-C due to its improved accuracy [48].
Does the Quantity of LDL Confer its Role as a Risk Factor?
The Framingham study was the first to report the association between LDL and CVD [49]. Subsequent studies have consistently found an inverse relationship between LDL (low-density lipoprotein) levels and the risk of heart disease. The management and reduction of LDL levels have emerged as a recommended approach by several organizations to mitigate the risk of CVD [18]. Numerous drugs have been discovered and approved for human use to lower lipid levels [4]. The fasting lipid profile test has become an essential component in the assessment of individuals with CV [9]. The link between LDL and CVD has been firmly established in the scientific and medical community from that time until the present day [30]. However, in recent years, emerging studies suggest that traditional LDL measurement factors such as particle size, density, and particle number may be better predictors of CVD than the traditional focus on LDL measurement [50-52].
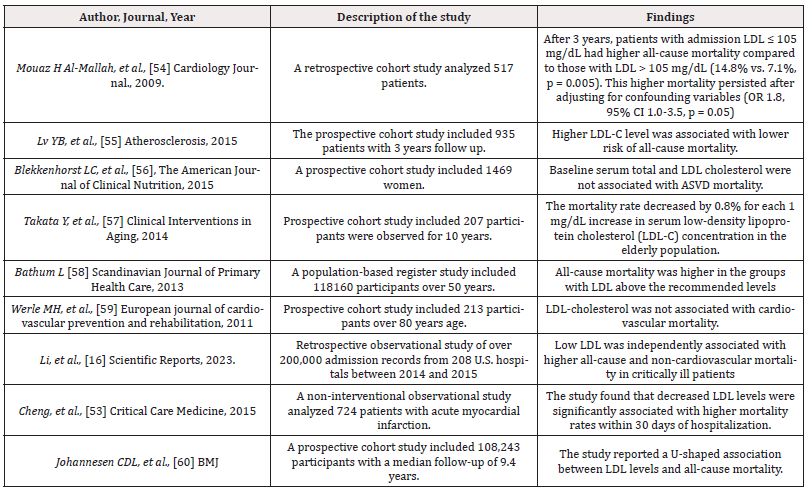
In their analysis of 27,316 hospitalized individuals, Li, et al. [16] reported a J-shaped association between LDL and all-cause mortality, with low LDL levels being associated with an increased risk for both all-cause mortality and non-cardiovascular mortality. However, there was no significant association between low LDL and cardiovascular mortality but interestingly, when low LDL was combined with low HDL (high-density lipoprotein), the risk increased for all-cause mortality, cardiovascular mortality, and non-cardiovascular mortality [16]. Cheng, et al. [53] conducted an observational study using a sample size of 724 patients hospitalized with acute myocardial infarction and the investigation revealed a significant association between decreased levels of LDL-C and increased mortality rates within 30 days of hospitalization (Table 1).
In a prospective cohort study conducted in Denmark [60], researchers analyzed 11,376 deaths and discovered a U-shaped association between LDL levels and all-cause mortality, challenging the notion that low LDL is inversely associated with increased CVD risk and mortality. Interestingly, the lowest risk for all-cause mortality was found at an LDL-C concentration of 3.6 mmol/L (140 mg/dL), which is above what is the a clinician target goal of below 100 mg/dL [60]. A prospective cohort study identified LDL Apo-B100 as a more accurate indicator of Myocardial Infarction (MI) compared to LDL, triglycerides (TGs), or cholesterol levels [61]. Surprisingly, among Chinese elderly, higher LDL-C level was associated with lower risk of all-cause mortality [55].
The conflicting results from different studies and various counterintuitive findings of measuring traditional LDL for cardiovascular disease risk assessment and management has caused clinicians and scientists to consider other means of LDL measurement. Researchers reported several alternative methods of measuring LDL includes LDL particle number, Apolipoprotein-B100, Small Dense LDL (sdLDL), LDL particle size, and LDL-C/ApoB ratio. Evidence suggests that these parameters may serve better than traditional measurement of LDL for cardiovascular disease mortality risk assessment and prevention [51,62].
LDL Particle Number
Standard lipid tests do not assess the count of LDL particles (LDL-P); rather, they measure the amount of LDL cholesterol [62]. LDL-P is a direct count of the number of LDL particles and refers to the actual count of low-density lipoprotein particles circulating in the bloodstream [62]. This is distinct from LDL-C, which quantifies the amount of cholesterol contained within LDL particles. LDL-P is often considered a more robust indicator of cardiovascular events compared to LDL-C [43].
It is possible to have a normal cholesterol concentration, or LDL-C, but still have a high number of LDL particles, or elevated LDL-P [63] which could potentially indicate a higher cardiovascular risk [63]. However, even with a high LDL-C, if your LDL-P is low, it could suggest a lower risk of cardiovascular events which highlights the importance of considering both LDL-P and LDL-C in assessing cardiovascular health [50]. CVD was increase 2.3 times more in top tertile HDL-P group in comparison with lower tertile group even after adjusted for LDL [64]. The JUPITER study reports that baseline LDL particle number was associated with CVD events, whereas LDL cholesterol levels were not [65].
Apolipoprotein-B100
Every LDL particle carries a single Apo-B100 molecule, and it is noteworthy that nearly 90% of all Apo-B100 molecules are transported by LDL [66]. The 2021 guidelines from the Canadian Cardiovascular Society on dyslipidemia suggest that healthcare providers can use apolipoprotein B (apoB) levels as alternatives to LDL cholesterol for both screening and treatment objectives [67]. According to a prospective cohort study, Apo-B100 has been identified as a more accurate indicator of Myocardial Infarction (MI) compared to LDL, TGs, or cholesterol levels [61]. Amusat, et al. reported in their study of 494 participants that Apo B exhibits high diagnostic accuracy, making it a suitable ASCVD risk biomarker [68]. Johannesen CDL et al. [60] reported that higher levels of excess apoB are dose-dependently associated with increased Hazard Ratios (HRs) for Myocardial Infarction (MI) and Atherosclerotic Cardiovascular Disease (ASCVD) in both women and men, indicating a greater risk of adverse cardiovascular events and mortality for individuals with elevated excess apoB [69]. The findings imply that measuring apoB levels could enhance the prediction and management of cardiovascular risk, especially in identifying high-risk individuals that LDL-C levels alone might not detect. A similar study reported that higher incidence of major adverse cardiovascular events (MACE; coronary heart disease death, nonfatal myocardial infarction, fatal/nonfatal ischemic stroke, hospitalization for unstable angina) was discovered in group with higher initial Apo-B level even after considering LDL cholesterol levels and MACE [70].
Small, Dense LDL
Small dense LDL are a subtype of LDL particles that are smaller and denser compared to larger more buoyant LDL particles. These particles are more atherogenic, meaning they have a higher potential to promote the formation of fatty deposits in the arteries, leading to cardiovascular diseases. Research indicates that small dense LDL cholesterol is a highly effective marker for predicting the residual risk of future Cardiovascular Events (CE) in patients with stable Coronary Artery Disease (CAD) who are on statin therapy, as well as in high-risk CAD patients who have diabetes or elevated triglyceride levels [71,72]. In a study involving a Japanese population, estimated small dense LDL was identified as an independent risk factor for revascularization and the development of new lesions in patients within two years following Percutaneous Coronary Intervention (PCI) for stable angina who were receiving statin therapy [73]. Another study shows that Small LDL particles are associated with an increased risk of CVD events, independent of LDL, and they predict events in both low-risk and very high-risk individuals [65].
LDL-C/Apob Ratio
The LDL-C/ApoB ratio serves as a convenient and accessible indicator of LDL particle size in clinical practice and the LDL-C/ApoB ratio independently predicts MACE in individuals with established atherosclerosis [74]. A study on US population reported LDL-C/ApoB ratio as an independent predictor for cardio-vascular and all-cause mortality [50]. Coronary heart disease continues to occur even in individuals with controlled Type-2 Diabetes (T2D) but rather than the atherogenic index of plasma, the LDL-C/ApoB ratio of ≤1.2 can predict coronary heart disease independently of the ASCVD risk score in T2D patients [75]. An observational study reported that even with normal LDL cholesterol levels, the LDL-C/ApoB ratio can still predict coronary atherosclerosis in non-diabetic patients [76].
Conclusion
Recent research findings suggest that the size and density of LDL particles may be more predictive than conventional LDL-C measurements for predicting CVD risk and mortality. Recent studies suggest a shift in the trend from measuring quantity to measuring quality of LDL, which includes the particle number of LDL, Apo-B100, LDL-C/ApoB ratio, and subclasses of LDL. Emerging technologies such as NMR (nuclear magnetic resonance) enhance the accessibility of these measurements. In the coming years, there may be a significant emphasis on assessing the quality of LDL
Acknowledgement
None.
Conflict of Interest
None to declare.
References
- Roth GA, Mensah GA, Johnson CO, Giovanni Addolorato, Enrico Ammirati, et al. (2020) Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019. J Am Coll Cardiol 76(25): 2982-3021.
- Martin SS, Aday AW, Almarzooq ZI, Cheryl Anderson AM, Pankaj Arora, et al. (2024) s2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 149(8): e347-e913.
- Severino P, D’Amato A, Pucci M, Fabio Infusino 1, Francesco Adamo, et al. (2020) Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int J Mol Sci 21(21): 8118.
- Wilkinson MJ, Lepor NE, Michos ED (2023) Evolving Management of Low‐Density Lipoprotein Cholesterol: A Personalized Approach to Preventing Atherosclerotic Cardiovascular Disease Across the Risk Continuum. J Am Heart Assoc 12(11): e028892.
- Alloubani A, Nimer R, Samara R (2021) Relationship between Hyperlipidemia, Cardiovascular Disease and Stroke: A Systematic Review. Curr Cardiol Rev 17(6): e051121189015.
- Mortensen MB, Nordestgaard BG (2020) Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70-100 years: a contemporary primary prevention cohort. The Lancet 396(10263): 1644-1652.
- Visseren FLJ, Mach F, Smulders YM, David Carballo, Konstantinos C Koskinas, et al. (2021) 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J 42(34) :3227-3337.
- Grundy SM, Stone NJ, Bailey AL, Craig Beam, Kim K Birtcher, et al. (2019) 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 73(24): e285-e350.
- Virani SS, Newby LK, Arnold SV, Vera Bittner, LaPrincess C Brewer, et al. (2023) 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 148(9):e9-e119.
- (2024) What Your Cholesterol Levels Mean. www.heart.org.
- Su X, Kong Y, Peng D (2019) Evidence for changing lipid management strategy to focus on non-high density lipoprotein cholesterol. Lipids Health Dis 18(1): 134.
- Sabatine MS, Wiviott SD, Im K, Murphy SA, Giugliano RP (2018) Efficacy and Safety of Further Lowering of Low-Density Lipoprotein Cholesterol in Patients Starting With Very Low Levels: A Meta-analysis. JAMA Cardiol 3(9): 823-828.
- Penson PE, Pirro M, Banach M (2020) LDL-C: lower is better for longer—even at low risk. BMC Med 18(1): 320.
- Karagiannis AD, Mehta A, Dhindsa DS, Salim S Virani, Carl E Orringer, et al. (2021) How low is safe? The frontier of very low (<30 mg/dL) LDL cholesterol. Eur Heart J 42(22): 2154-2169.
- Faselis C, Imprialos K, Grassos H, Pittaras A, Kallistratos M, et al. (2018) Is very low LDL-C harmful? Curr Pharm Des 24(31): 3658-3664.
- Li S, Zhang W, Liu H (2013) Association between lipid levels and all-cause and cause-specific mortality in critically ill patients. Sci Rep 13(1): 5109.
- Ennezat PV, Guerbaai RA, Maréchaux S, Le Jemtel TH, François P (2023) Extent of Low-density Lipoprotein Cholesterol Reduction and All-cause and Cardiovascular Mortality Benefit: A Systematic Review and Meta-analysis. J Cardiovasc Pharmacol 81(1): 35.
- Byrne P, Demasi M, Jones M, Smith SM, O’Brien KK, et al. (2022) Evaluating the Association Between Low-Density Lipoprotein Cholesterol Reduction and Relative and Absolute Effects of Statin Treatment: A Systematic Review and Meta-analysis. JAMA Intern Med 182(5): 474-481.
- Gofman JW, Rubin L, McGINLEY JP, Jones HB (1954) Hyperlipoproteinemia. Am J Med 17(4): 514-520.
- Havel RJ (2007) Introduction: John Gofman and the early years at the Donner Laboratory. J Clin Lipidol 1(2): 100-103.
- Goldstein JL, Brown MS (1973) Familial Hypercholesterolemia: Identification of a Defect in the Regulation of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Activity Associated with Overproduction of Cholesterol. Proc Natl Acad Sci 70(10): 2804-2808.
- Gofman JW, Delalla O, Glazier F, Norman K Freeman, Frank T Lindgren, et al. (2007) The serum lipoprotein transport system in health, metabolic disorders, atherosclerosis and coronary heart disease. J Clin Lipidol 1(2): 104-141.
- Alberts AW, Chen J, Kuron G, V Hunt, J Huff, et al. (1980) Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci 77(7): 3957-3961.
- Uauy R, Vega GL, Grundy SM, Bilheimer DM (1988) Lovastatin therapy in receptor-negative homozygous familial hypercholesterolemia: Lack of effect on low-density lipoprotein concentrations or turnover. J Pediatr 113(2): 387-392.
- Xiao C, Stahel P, Nahmias A, Lewis GF (2020) Emerging Role of Lymphatics in the Regulation of Intestinal Lipid Mobilization. Front Physiol 10: 1604.
- Li X, Liu Q, Pan Y, Chen S, Zhao Y, et al. (2023) New insights into the role of dietary triglyceride absorption in obesity and metabolic diseases. Front Pharmacol 14: 1097835.
- Amadi PU, Zhang DW (2024) Cholesin, a new hormone bridges intestinal cholesterol absorption and hepatic synthesis. Life Metab 3(4): loae024.
- Keirns BH, Sciarrillo CM, Koemel NA, Emerson SR (2021) Fasting, non-fasting and postprandial triglycerides for screening cardiometabolic risk. J Nutr Sci 10: e75.
- Prassl R, Laggner P (2009) Molecular structure of low-density lipoprotein: current status and future challenges. Eur Biophys J 38(2): 145-158.
- Tall AR, Thomas DG, Gonzalez Cabodevilla AG, Goldberg IJ (2022) Addressing dyslipidemic risk beyond LDL-cholesterol. J Clin Invest 132(1): e148559.
- Mundi S, Massaro M, Scoditti E, Maria Annunziata Carluccio, Victor WM Van Hinsbergh, et al. (2018) Endothelial permeability, LDL deposition, and cardiovascular risk factors—a review. Cardiovasc Res 114(1): 35-52.
- Rauschert S, Gázquez A, Uhl O, Franca F Kirchberg, Hans Demmelmair, et al. (2019) Phospholipids in lipoproteins: compositional differences across VLDL, LDL, and HDL in pregnant women. Lipids Health Dis 18(1): 20.
- Hevonoja T, Pentikäinen MO, Hyvönen MT, Kovanen PT, Ala Korpela M (2000) Structure of low-density lipoprotein (LDL) particles: Basis for understanding molecular changes in modified LDL. Biochim Biophys Acta BBA - Mol Cell Biol Lipids 1488(3): 189-210.
- Zampelas A, Magriplis E (2019) New Insights into Cholesterol Functions: A Friend or an Enemy? Nutrients 11(7): 1645.
- Venugopal SK, Anoruo M, Jialal I (2023) Biochemistry, Low Density Lipoprotein. In: StatPearls.
- Jebari Benslaiman S, Galicia García U, Larrea Sebal A, Javier Rekondo Olaetxea, Iraide Alloza, et al. (2022) Pathophysiology of Atherosclerosis. Int J Mol Sci 23(6): 3346.
- Minelli S, Minelli P, Montinari MR (2020) Reflections on Atherosclerosis: Lesson from the Past and Future Research Directions. J Multidiscip Healthc 13: 621-633.
- Kowara M, Cudnoch-Jedrzejewska A (2021) Pathophysiology of Atherosclerotic Plaque Development-Contemporary Experience and New Directions in Research. Int J Mol Sci 22(7): 3513.
- Babaniamansour P, Mohammadi M, Babaniamansour S, Aliniagerdroudbari E (2020) The Relation between Atherosclerosis Plaque Composition and Plaque Rupture. J Med Signals Sens 10(4): 267-273.
- Chary A, Hedayati M (2022) Review of Laboratory Methods to Determine HDL and LDL Subclasses and Their Clinical Importance. Rev Cardiovasc Med 23(4): 147.
- Hirayama S, Miida T (2012) Small dense LDL: An emerging risk factor for cardiovascular disease. Clin Chim Acta 414:215-224.
- Kanonidou C (2021) Small dense low-density lipoprotein: Analytical review. Clin Chim Acta 520: 172-178.
- Qiao YN, Zou YL, Guo SD (2022) Low-density lipoprotein particles in atherosclerosis. Front Physiol 13: 931931.
- Monsonis Centelles S, Hoefsloot HCJ, Khakimov B, Parvaneh Ebrahimi, Mads Lind V, et al. (2017) Toward Reliable Lipoprotein Particle Predictions from NMR Spectra of Human Blood: An Interlaboratory Ring Test. Anal Chem 89(15): 8004-8012.
- Sajja A, Park J, Sathiyakumar V, Bibin Varghese, Vincent A Pallazola, et al. (2021) Comparison of Methods to Estimate Low-Density Lipoprotein Cholesterol in Patients With High Triglyceride Levels. JAMA Netw Open 4(10): e2128817.
- Wang I, Rahman MH, Hou S, Lin HW (2023) Assessing the Practical Differences in LDL-C Estimates Calculated by Friedewald, Martin/Hopkins, or NIH Equation 2: An Observation Cross-Sectional Study. J Lipid Atheroscler 12(3): 252-266.
- Martin SS, Blaha MJ, Elshazly MB, Peter P Toth, Peter O Kwiterovich, et al. (2013) Comparison of a Novel Method vs the Friedewald Equation for Estimating Low-Density Lipoprotein Cholesterol Levels From the Standard Lipid Profile. JAMA 310(19): 2061-2068.
- Grant JK, Kaufman HW, Martin SS (2024) Extensive Evidence Supports the Martin-Hopkins Equation as the LDL-C Calculation of Choice. Clin Chem 70(2): 392-398.
- Gordon T, Kannel WB, Castelli WP, Dawber TR (1981) Lipoproteins, Cardiovascular Disease, and Death: The Framingham Study. Arch Intern Med 141(9):1128-1131.
- Xiao L, Zhang K, Wang F, Min Wang, Qingxia Huang, et al. (2023) The LDL-C/ApoB ratio predicts cardiovascular and all-cause mortality in the general population. Lipids Health Dis 22(1): 104.
- Pichler G, Amigo N, Tellez Plaza M, Pardo Cea MA, Dominguez Lucaset A, al. (2018) LDL particle size and composition and incident cardiovascular disease in a South-European population: The Hortega-Liposcale Follow-up Study. Int J Cardiol 264: 172-178.
- Ikezaki H, Lim E, Cupples LA, Liu C, Asztalos BF, et al. (2021) Small Dense Low‐Density Lipoprotein Cholesterol Is the Most Atherogenic Lipoprotein Parameter in the Prospective Framingham Offspring Study. J Am Heart Assoc 10(5): e019140.
- Cheng KH, Chu CS, Lin TH, Lee KT, Sheu SH, et al. (2015) Lipid Paradox in Acute Myocardial Infarction—The Association With 30-Day In-Hospital Mortality. Crit Care Med 43(6): 1255-1264.
- Al Mallah MH, Hatahet H, Cavalcante JL, Khanal S (2009) Low admission LDL-cholesterol is associated with increased 3-year all-cause mortality in patients with non-ST segment elevation myocardial infarction. Cardiol J 16(3): 227-233.
- Lv YB, Yin ZX, Chei CL, Han Zhu Qian, Virginia Byers Kraus, et al. (2015) Low-density lipoprotein cholesterol was inversely associated with 3-year all-cause mortality among Chinese oldest old: Data from the Chinese Longitudinal Healthy Longevity Survey. Atherosclerosis 239(1): 137-142.
- Blekkenhorst LC, Prince RL, Hodgson JM, Wai H Lim, Kun Zhu, et al. (2015) Dietary saturated fat intake and atherosclerotic vascular disease mortality in elderly women: a prospective cohort study. Am J Clin Nutr 101(6): 1263-1268.
- Takata Y, Ansai T, Soh I, Shuji Awano, Ikuo Nakamichi, et al. (2014) Serum total cholesterol concentration and 10-year mortality in an 85-year-old population. Clin Interv Aging 9: 293-300.
- Bathum L, Depont Christensen R, Engers Pedersen L, Lyngsie Pedersen P, Larsen J, et al. (2013) Association of lipoprotein levels with mortality in subjects aged 50+ without previous diabetes or cardiovascular disease: A population-based register study. Scand J Prim Health Care 31(3):172-180.
- Werle MH, Moriguchi E, Fuchs SC, Bruscato NM, de Carli W, et al. (2011) Risk factors for cardiovascular disease in the very elderly: results of a cohort study in a city in southern Brazil. Eur J Cardiovasc Prev Rehabil 18(3): 369-377.
- Johannesen CDL, Langsted A, Mortensen MB, Nordestgaard BG (2020) Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ 371: m4266.
- Marston NA, Giugliano RP, Melloni GEM, Jeong Gun Park, Valerie Morrill, et al. (2022) Association of Apolipoprotein B-Containing Lipoproteins and Risk of Myocardial Infarction in Individuals With and Without Atherosclerosis: Distinguishing Between Particle Concentration, Type, and Content. JAMA Cardiol 7(3): 250-256.
- Sykes AV, Patel N, Lee D, Taub PR (2022) Integrating Advanced Lipid Testing and Biomarkers in Assessment and Treatment. Curr Cardiol Rep 24(11): 1647-1655.
- Hassan S, Triffon DW (2024) Unlocking the lipid puzzle: deciphering discordance among ldl, non-hdl, ldl-p, and apob values and their impact on cardiovascular risk assessment. J Am Coll Cardiol 83(13_Supplement): 1849-1849.
- Melander O, Shiffman D, Caulfield MP, Judy Louie Z, Charles Rowland M, et al. (2015) Low-Density Lipoprotein Particle Number Is Associated With Cardiovascular Events Among Those Not Classified Into Statin Benefit Groups. J Am Coll Cardiol 65(23): 2571-2573.
- Shiffman D, Louie JZ, Caulfield MP, Nilsson PM, Devlin JJ, et al. (2017) LDL subfractions are associated with incident cardiovascular disease in the Malmö Prevention Project Study. Atherosclerosis 263: 287-292.
- Jeiran K, Gordon SM, Sviridov DO, Angel Aponte M, Amanda Haymond, et al. (2022) A New Structural Model of Apolipoprotein B100 Based on Computational Modeling and Cross Linking. Int J Mol Sci 23(19): 11480.
- Pearson GJ, Thanassoulis G, Anderson TJ, Arden R Barry 4, Patrick Couture, et al. (2021) 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can J Cardiol 37(8): 1129-1150.
- Amusat S (2024) Comparative Assessment of hsCRP and Apolipoprotein B as ASCVD Risk Biomarkers. J Clin Lipidol 18(4): e499.
- Johannesen CDL, Langsted A, Nordestgaard BG, Mortensen MB (2024) Excess Apolipoprotein B and Cardiovascular Risk in Women and Men. J Am Coll Cardiol 83(23): 2262-2273.
- Hagström E, Steg PG, Szarek M, Deepak Bhatt L, Vera Bittner A, et al. (2022) Apolipoprotein B, Residual Cardiovascular Risk After Acute Coronary Syndrome, and Effects of Alirocumab. Circulation 146(9): 657-672.
- Sakai K, Koba S, Nakamura Y, Yuya Yokota, Fumiyoshi Tsunoda, et al. (2018) Small dense low-density lipoprotein cholesterol is a promising biomarker for secondary prevention in older men with stable coronary artery disease. Geriatr Gerontol Int 18(6): 965-972.
- Krychtiuk KA, Kastl SP, Pfaffenberger S, Max Lenz, et al. (2015) Association of Small Dense LDL Serum Levels and Circulating Monocyte Subsets in Stable Coronary Artery Disease. PLOS ONE 10(4): e0123367.
- Kanda D, Tokushige A, Ohishi M (2024) Association between Estimated Small Dense Low-Density Lipoprotein Cholesterol and Occurrence of New Lesions after Percutaneous Coronary Intervention in Japanese Patients with Stable Angina and Receiving Statin Therapy. Rev Cardiovasc Med 25(6): 218.
- Drexel H, Larcher B, Mader A, Alexander Vonbank, Christine F Heinzle, et al. (2021) The LDL-C/ApoB ratio predicts major cardiovascular events in patients with established atherosclerotic cardiovascular disease. Atherosclerosis 329: 44-49.
- Rabizadeh S, Rajab A, Mechanick JI, Fatemeh Moosaie, Yekta Rahimi, et al. (2021) LDL/apo B ratio predict coronary heart disease in Type 2 diabetes independent of ASCVD risk score: A case-cohort study. Nutr Metab Cardiovasc Dis 31(5): 1477-1485.
- Jung HW, Ra M, Bae HJ, Hong SP (2023) The LDL-C/Apo B predicts coronary atherosclerotic heart disease in non-diabetic patients without high LDL-C. Medicine (Baltimore) 102(1): e32596.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.